Many users who want to incorporate text analytics into their Voice of the Employee (VoE) analysis aren’t sure how to translate their business requirements into something compatible with their current processes.

Many users who want to incorporate text analytics into their Voice of the Employee (VoE) analysis aren’t sure how to translate their business requirements into something compatible with their current processes.
At MeaningCloud, we are proud to sponsor the PharmaForce 2017 Conference. PharmaForce is an interactive conference for marketing and sales leaders from pharmaceutical companies to be held on September 18-20, 2017, in Austin, TX.
PharmaForce is the event for Pharma commercial innovators.
Who should attend:
Now in its 11th year, PharmaForce is the only life sciences event that addresses both marketing and sales strategies to achieve commercial success.
In a previous post (Listening to the Voice of the Patient), we made an account of ongoing initiatives by public administration, hospitals and pharma companies intended to listen, understand and engage patients in the whole healthcare system. We also provided evidence that forums are a paramount source of information when talking about the Voice of the Patient Analysis.
As it was already shown, the Voice of the Patient (VoP) can be analyzed from multiple perspectives. In this post, we cover the point of view of the Pharma Industry.


Since everyone wants to understand employees better, text-based data sources are a key factor for any organization to understand the “whys” and act on them to make improvements. Open-ended questions are one of the most effective ways to gather employee opinions; they offer them an open forum to make suggestions and present innovative ideas.
The modern definition of “patient centered health care” was stated in the National Library of Medicine’s MED-LINE subject heading (MeSH), introduced in 1995, which reads, “Design of patient care wherein institutional resources and personnel are organized around patients rather than around specialized departments.”
Following this design criterion, patients’ safety and well-being are the priority for all the agents involved in this industry: caregivers, pharmaceutical companies, medical device manufacturers, health insurers, and government agencies. And, being the center of our health systems, listening and engaging patients becomes the cornerstone of any quality improvement initiative. That’s why the so called “Voice of the Patient” is getting an increasing attention by all the stakeholders involved.
Real World Evidence is information on health care that is derived from multiple sources outside typical clinical research settings, including electronic health records (EHRs), claims and billing data, product and disease registries, and data gathered through personal devices and health applications.
The most quoted definition of Real World Data comes from the area of pharmacoeconomics. The ISPOR (International Society for Pharmacoeconomics and Outcomes Research) defines Real World Data as:
“Data used for decision making that are not collected in conventional randomized controlled trials (RCTs)”
Other definitions of Real World Data, Real World Evidence and Evidence from Clinical Experience can be found in the following figure, taken from a working paper of the Margolis Center for Health Policy, Duke University (see References below).

Real-World Evidence Forum Philadelphia, July 17-18
At MeaningCloud, we are proud to sponsor the Real World Evidence Forum. The RWE Forum, taking place on July 17-18, 2017 in Philadelphia, will bring together clinical health professionals to address:
Attendees will gain a better understanding of how electronic data sources are changing the way real-world data is being collected. This conference will offer attendees insight into how real-world evidence will help decrease costs, define innovative outcomes and minimize the number of patients exposed to potentially harmful medications.
MeaningCloud, as a Text Analytics provider, has evolved a highly specialized offering for the Health and Pharma industries. We count among our clients some the largest companies in the Pharmaceutical industry.
Join us in Philadelphia. If you are interested in attending the Real World Evidence Forum next July 17-18, just drop us a line to info@meaningcloud.com. We have a surprise for you!
Stay tuned to access our presentation at the conference, that we will publish on this blog. In the meanwhile, if you are curious about how our technology works in the health area, just take a look at our Text Analytics Health Demo.
Looking forward to seeing you at the Real-World Evidence Forum!

Real World Evidence (AKA “Real World Data”) is a worldwide trend in Health and Life Sciences. New kinds of data, such as electronic health records and data mining tools are now available and allow us to extract information and knowledge. We can detect medical treatment costs, treatment efficiency (cost, benefits, and risks), references to drugs, side effects, or long-term results. Text analytics is an essential component of this area of knowledge.
Austerity measures and related price cuts have put unprecedented pressure on the pharmaceutical industry. Manufacturers are being asked to provide information related not only to safety, appropriate use, and effectiveness but also to clinical and economic value. Although randomized clinical trials (RCTs) remain the gold standard of clinical tests, factors such as varying responses to a drug in real life, not completing the course of prescriptions, or using unauthorized medication before or during the trial limit the generalizability of results from randomized clinical trials.
Real World Evidence (also called “Real World Data”) has been fueled by new data technologies that leverage the valuable information contained in electronic medical records and personal information repositories. This post is a review of those Real World Evidence sources and of the benefits that Pharmaceutical and Life Science companies can derive from them.
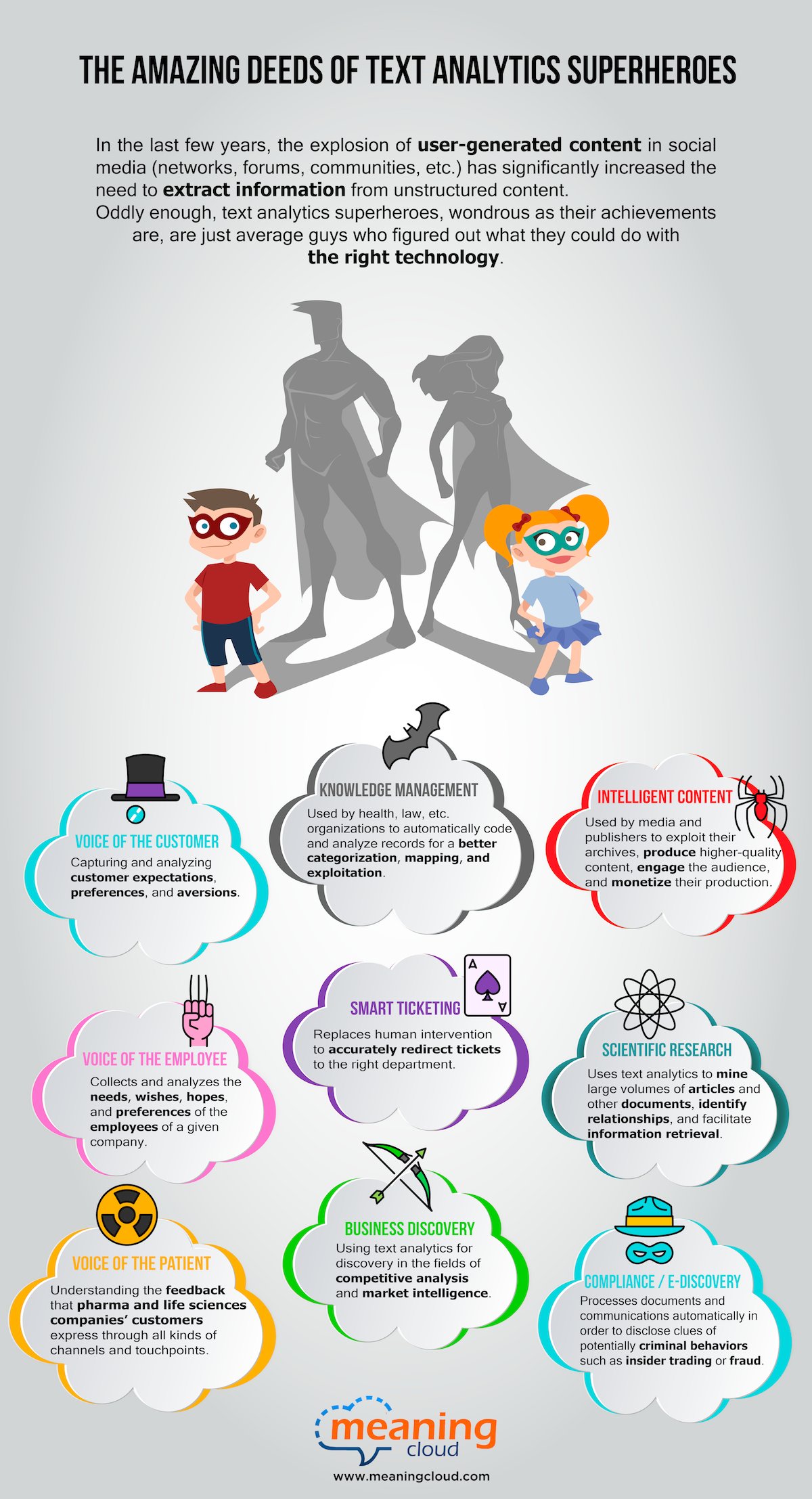
In the last few years, the explosion of user-generated content in social media (networks, forums, communities, etc.) has significantly increased the need to extract information from unstructured content. Oddly enough, text analytics superheroes, wondrous as their achievements are, are just average guys who figured out what they could do with the right technology.
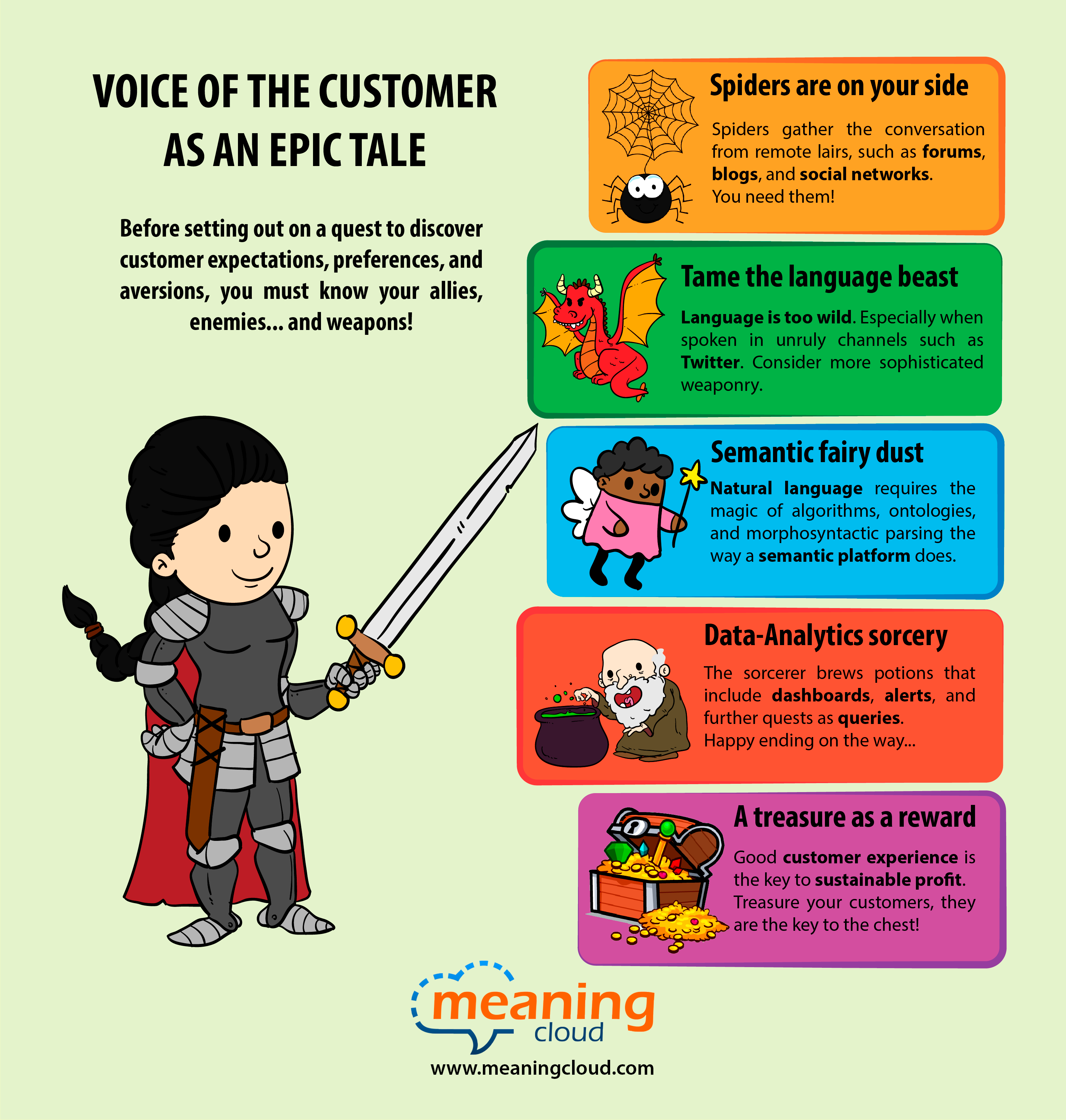
Before setting out on a quest to discover customer expectations, preferences, and aversions, you must know your allies, enemies, and weapons.
But, be on guard, since dangers lurk in the horizon…