
For the pharmaceutical industry, it is essential to listen and understand the feedback that their current and potential patients communicate through all sorts of channels and touchpoints.
Although there is a protocol that requires any identified Adverse Drug Reactions (ADRs) to be disclosed to the authorities, only 5–20% of them are reported. Fortunately, discussions regarding drugs, symptoms, conditions, and diseases can be analyzed to learn more about said branches of pharmaceutics. Artificial Intelligence significantly contributes in monitoring adverse episodes and understanding their impact in every phase of development.
Patient narratives of medicines and their adverse effects on social media represent an extra data source for drug safety monitoring.
At MeaningCloud, we have developed a platform to automate the process of monitoring ADRs on social media.
Adverse Drug Reactions: A growing problem
Evidence continues to grow that shows adverse reactions to medicines are a common source of illness, disability, and even death.
In some countries, adverse drug reactions (ADRs) are rated among the top 10 leading causes of mortality. Aside from the more general inherent dangers associated with drugs, individual patients may also present unusual and unpredictable sensitivities to certain medicines.
Similarly, if more than one medicine is prescribed, there is always a risk of negative interactions. The selection and use of the best and safest medicine(s) for any given individual out of the many possibilities available thus requires considerable skill on behalf of the prescribing practitioner.
That is why pharmaceutical brands are extending their Voice of the Patient initiatives toward a new territory of unsolicited and unstructured content: comments on surveys, call center verbatims, Twitter, etc.
The process, which uses natural language processing (NLP) techniques to interpret vast quantities of text quickly, can change every step of the drug development process.
Finding relevant information in large volumes of unstructured text using a conventional keyword search can be an arduous task.
We have now a dedicated business exclusively focused on the health and pharmaceutical sectors
Konplik.Health begins operations with the health-related assets from MeaningCloud, including its leading natural language processing, deep semantic analysis, AI platform, and adaptations specific to the life sciences.
How does the MeaningCloud Pharmacovigilance platform work
Text Analytics makes the automatic processing, tagging, and extraction of insights possible among the aforementioned pharmaceutical branches.
- It all begins by defining the terms that we have a personal interest in monitoring. Regularly they are drug names, such as “Ovitrelle” or “Puregon”.
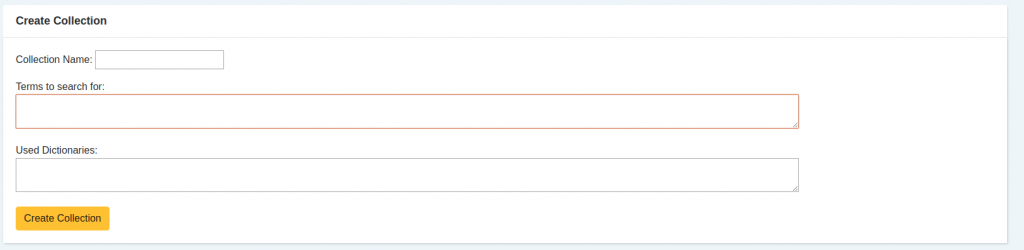
In addition to these customization capabilities, the tool includes lexical resources from the health domain, allowing for the identification of drugs, active principles, adverse reactions, and other health-related taxonomies. These lexical resources include a link to well-known dictionaries like SNOMED CT, ICD or MedDRA.
- Then the crawlers go in search of the words. They collate them along with the context in which they appear. Then, each occurrence is processed using our semantic technology.

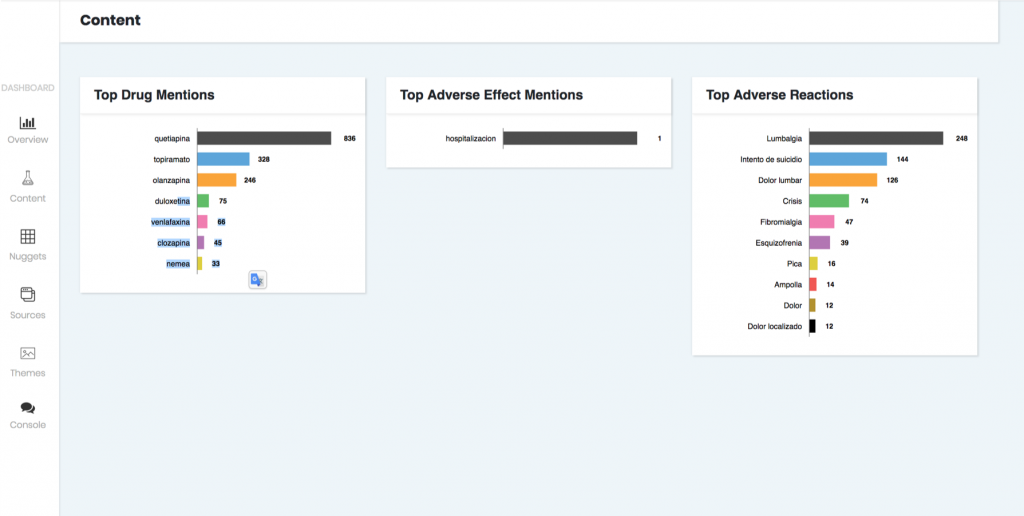
- Subsequently, the platform generates reports to better understand how adverse reactions and drug mentions are appearing in social media sources.
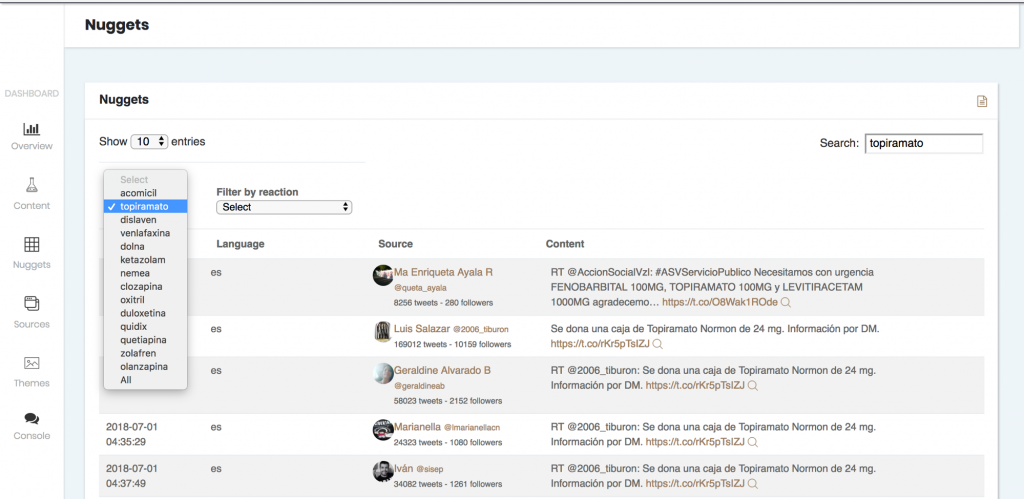
- It also allows you to refer back to the sources where each output appeared.
Should you be interested, we would willingly show you how this platform could be of assistance to you. Do not hesitate to contact us at support@meaningcloud.com.



One thought on “Pharmacovigilance: Monitoring the Voice of the Patient”
It is great to read such informative blogs. Thanks for sharing…!